Despite the notable progress that has been achieved in the realm of MM treatment, the persistence of elevated relapse rates and therapy resistance remain a substantial hindrance to effective management of the patients. Therefore, the development of modern tools to ameliorate patients' prognosis and predict treatment outcome is of first clinical priority. MicroRNAs (miRNAs) have emerged as powerful post-transcriptional regulators of gene expression, while recent advances in high-throughput sequencing techniques have unveiled their prognostic utility in numerous pathologies. Herein, using a miRNA-seq we have investigated miRNA profiles in association with Revised International Staging System (R-ISS), aiming to reveal potential MM-related miRNAs able to improve patients' risk stratification.
Bone marrow (BM) aspiration samples were collected from 120 MM patients at diagnosis (screening cohort). Mononuclear cells were isolated using Ficoll-Paque, while CD138 + plasma cells were positively selected using anti-CD138 mAbs magnetic beads. miRNA-seq was performed in CD138 + cells from 8 R-ISS I, 8 R-ISS II and 8 R-ISS III MM patients. Following 3‘-end polyadenylation of total RNA, miR-221/222 levels were quantified in our screening cohort by RT-qPCR. Disease progression (relapse and/or death) and patients’ mortality were assessed as clinical endpoint events, while internal validation was accomplished by bootstrap Cox proportional regression analysis. Aash et al. 2023 (n=86) and Kruykov et al. 2016 (n=149) served as external validation cohorts.
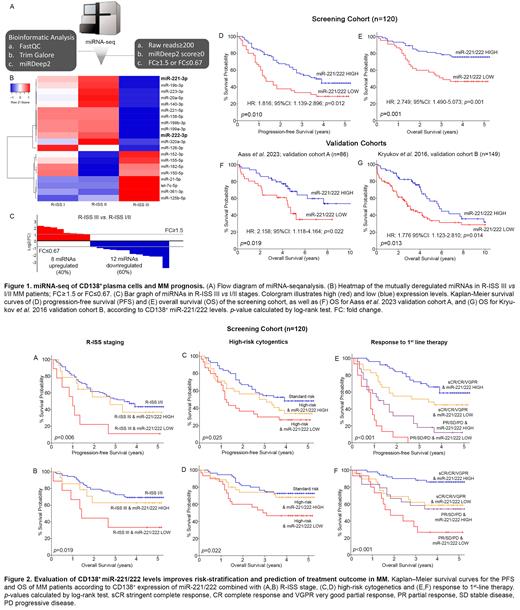
miRNA-seq revealed miR-221/222 to be concurrently downregulated in R-ISS III versus R-ISS I/II MM patients (miR-221: FC=0.356; miR-222: FC=0.357; Fig.1B,C). The analysis of our screening cohort documented that CD138 + miR-221/222 loss is significantly associated with higher risk for short-term disease progression ( p=0.010; Fig.1D) and poor survival ( p=0.001; Fig.1E) of MM patients. Furthermore, the analysis of the two independent validation cohorts (Aass et al. cohort A, p=0.019; and Kryukov et al. cohort B, p=0.013) clearly confirmed the worse treatment outcome of the patients with miR-221/222 loss (Fig.1F,G). Moreover, miR-221/222-fitted multivariate models demonstrated the ability of miR-221/222 cluster to ameliorate the clinical routine of the established MM prognostic markers, including “R-ISS stage”, “high-risk cytogenetics” [+1q21, t (4;14) del(17p13), t(14;16), t(11;14), del(13q)] and “response to 1 st line therapy” (IMWG guidelines). In this context, the evaluation of CD138 + miR-221/222 levels significantly improved the risk-stratification of MM patients, resulting in the advanced positive prediction of patients' poor treatment outcome within R-ISS III (PFS: p=0.006, OS: p=0.019; Fig.2A,B) and high-risk cytogenetics groups (PFS: p=0.025, OS: p=0.022; Fig.2C,D). Finally, focusing on patients' response to 1 st line therapy stratification, loss of miR-221/222 could efficiently define optimal (sCR/CR/VGPR) and poor (PR/SD/PD) responders' subgroups (PFS: p<0.001, OS: p<0.001; Fig.2E,F).
In conclusion, we identified miR-221/222 loss in CD138 + plasma cells as a powerful independent predictor of patients' risk for disease progression and poor treatment outcome, while miR-221/222-fitted multivariate prognostic models resulted to improved patients' risk-stratification and prediction of MM treatment outcome.
Disclosures
Gavriatopoulou:Celgene/Genesis: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria; X4 Pharmaceuticals: Research Funding; Karyopharm: Honoraria, Research Funding; Sanofi: Honoraria. Kastritis:GSK: Honoraria, Research Funding; Sanofi: Honoraria; Pfizer: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Terpos:BMS: Honoraria; GSK: Honoraria, Research Funding; Menarini/Stemline: Honoraria; ASTRA/Zeneca: Honoraria, Other: Travel Expenses; Sanofi: Honoraria, Other: Travel expenses, Research Funding; Pfizer: Honoraria; Takeda: Honoraria, Other: Travel expenses, Research Funding; Janssen: Honoraria, Research Funding; EUSA Pharma: Honoraria, Other: Travel expenses; Amgen: Honoraria, Other: Travel Expenses, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal